The Earth’s climate is a complex system influenced by various factors, and one of the most important pieces is greenhouse gases. These little guys—carbon dioxide (CO₂), water vapor (H₂O), methane (CH₄), and a few others—have a special talent: they trap heat from the sun, keeping our planet warm and cozy. But what makes some gases great at this job while others just float around doing nothing? Time to dive into the physics of greenhouse gases!
Mechanism
In our other discussions, we often referred to objects as “black bodies.” These are entities that efficiently absorb and emit various frequencies of infrared light. In most cases, we simplified our calculations by assuming an emissivity (ε) value close to one. But gases? Oh no, they play by totally different rules.
Gases are made of molecules that only vibrate in very specific ways, unlike solids or liquids, which have all kinds of jiggly motion going on. Take carbon dioxide (CO₂), for example. As dry ice (its solid form), CO₂ is a complex, vibrating mess. But as a gas? It sticks to a few very specific dance moves. This pickiness means gases only absorb and emit certain types of light.
For black bodies, ε close to one is a good assumption, but gases? Their ε values are much, much lower because they’re incredibly picky about which light waves they interact with.
Now, most of the colors we see around us come from electrons hopping between energy levels, absorbing or emitting light in the process. But gas molecules? They’re relatively simple, with big gaps between their electron energy levels. This means they mostly mess around with ultraviolet light, which we can’t see. There are a few exceptions, though—chlorine gas looks green, and nitrogen dioxide (NO₂) has that ugly brownish smog color.
Vibration
Anyway, for our purposes of understanding atmospheric energy dynamics, we return to molecular and atomic vibrations. For a gas molecule to absorb or emit light, it has to meet two criteria:
- The frequency of the light has to match the molecule’s vibration—like tuning a radio to the right station.
- The vibration must create a wiggling electric field, called an oscillating dipole.
Most gases in our atmosphere, like oxygen (O₂) and nitrogen (N₂), are made of two identical atoms stuck together symmetrically. Since they don’t have the right kind of electric field fluctuations, they’re practically invisible to infrared light—aka, they don’t act as greenhouse gases.
What makes a Greenhouse Gas special?
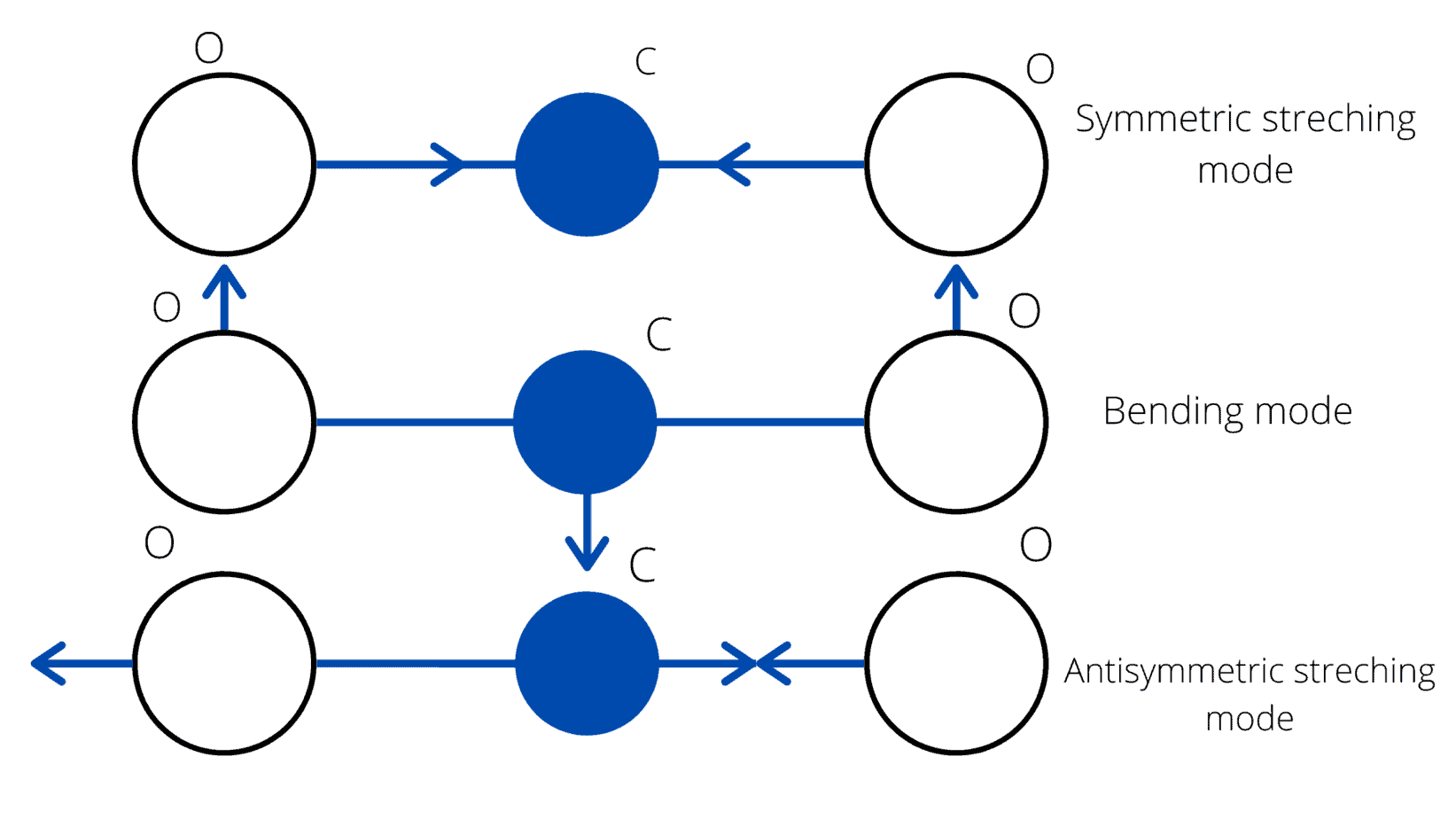
Now let’s talk about the real MVPs. CO₂ is a great example. In its resting state, it looks nice and symmetrical, with a carbon atom in the middle and two oxygens on either side. But when it starts vibrating, things get interesting.
Its most important dance move? The bending motion—this one breaks its symmetry and creates an oscillating dipole, making CO₂ a champion at absorbing infrared light from the Earth’s surface.
CO₂ also has two other ways it can vibrate: symmetric stretching (where everything moves in sync) and asymmetric stretching (where the oxygens move in opposite directions). The asymmetric stretch is “infrared active” (meaning it absorbs heat), but it’s not as important for climate science because it doesn’t line up with the most abundant heat wavelengths.
CO₂ isn’t alone in this game—water vapor (H₂O) and methane (CH₄) are also major players.
- Water vapor is naturally asymmetrical, thanks to the way its oxygen atom hogs electrons, pushing the hydrogen atoms to one side. This lopsided shape makes it ridiculously good at absorbing a wide range of infrared light, making it a powerhouse greenhouse gas.
- Methane (CH₄) looks symmetrical at first glance (with its neat tetrahedral shape), but it has enough funky vibrational modes to break symmetry, allowing it to absorb and trap heat effectively.
In fact, any molecule with more than two atoms has at least some potential to be a greenhouse gas! The physics behind it all is complex, but the impact is crystal clear: these gases help shape our planet’s climate. Understanding how they work is key to tackling climate change and figuring out how to keep our world livable for future generations.
Pretty cool, right? Or should I say… pretty hot? 🔥🌍
(… I swear I am way funnier in my native language idk why in English I could only make dad jokes)